Mission 2030
By 2030, we will fund and support more PSC research than ever before, offering over £1 million in grants to researchers to:
- fund PSC studies aligned with our Research Strategy, turning researchers’ ideas into reality;
- fund 3-4 year PhD clinical fellowships in PSC to help develop the careers of future PSC specialists and to fill the critical gaps in our knowledge about PSC; and
- support them to set up larger studies and clinical trials.
Apply for Funding
PSC Support welcomes grant applications for PSC-related research. As a member of the Association of Medical Research Charities, PSC Support employs strict peer review processes to ensure we fund only the highest quality research.
Available Awards
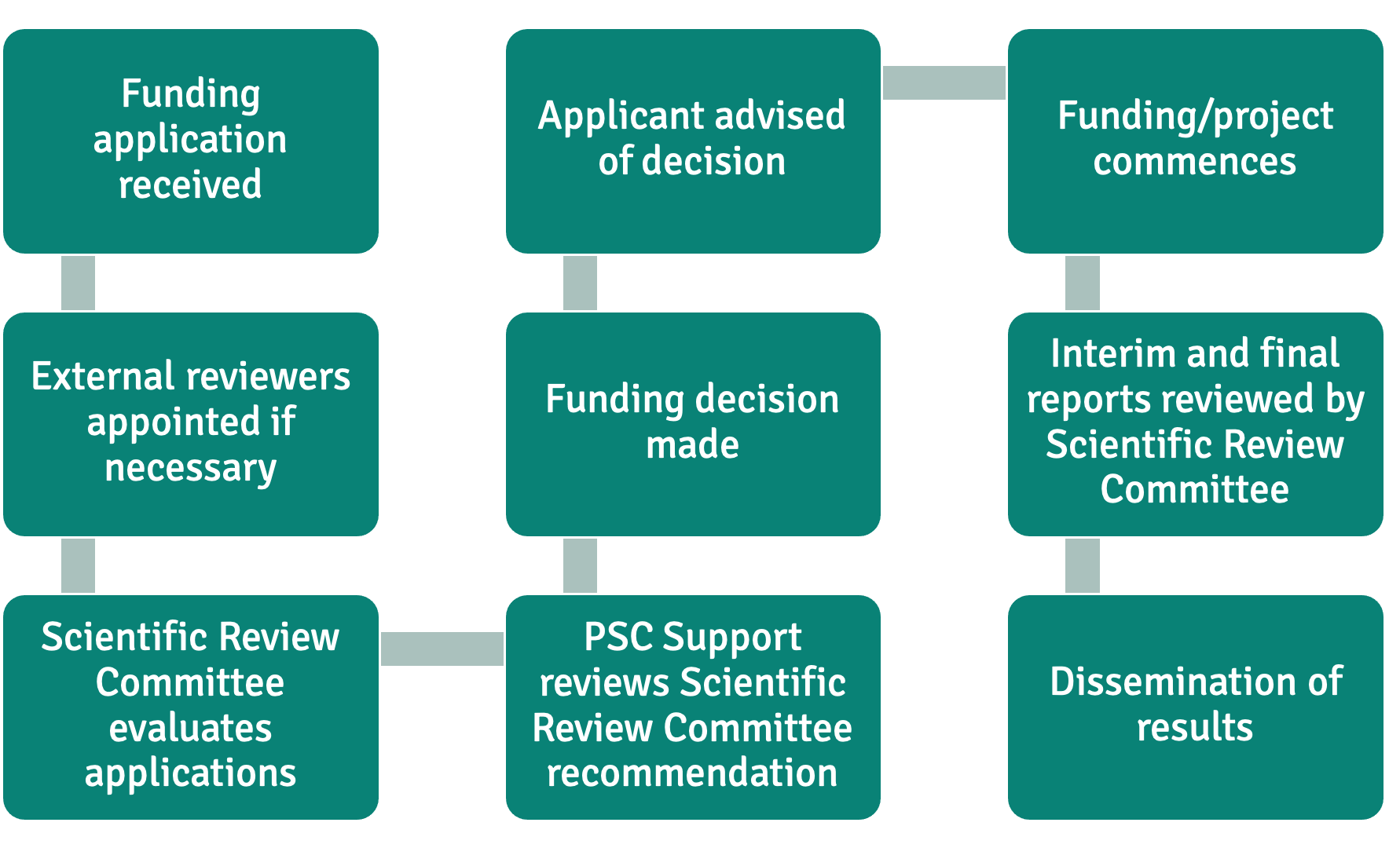
Our Research Funding Process
We aim to complete this process in two to three months.
Our Scientific Review Committee
Our Scientific Review Committee is responsible for evaluating and appraising research grant applications. They are independent of the charity's staff and trustees.
Dr Roger Chapman (Chair)
Chris Forde (lay)
Gareth Weeks (lay)

We've joined a select group of 150 UK charities that have demonstrated best practice in managing research funding and PSC Support is proud to have been approved as a member of the Association of Medical Research Charities (AMRC). The AMRC is a prestigious membership organisation dedicated to supporting medical research charities in saving and improving lives through research and innovation.
Martine Walmsley, PSC Support Head of Research Strategy, said, "To be an approved AMRC member is a real testament to our long-term commitment to invest in the very best PSC research and something we are incredibly proud to have achieved. All of PSC Support's funds come from donations. It is important that our incredible donors and fundraisers can be confident that the research they are helping to fund is not only high quality, but also part of a clear strategic plan towards developing effective treatments for PSC."
AMRC membership demonstrates that PSC Support delivers high-quality research and is an indicator of the excellent work we do in research governance and standards. All members, whatever their size, work to the same high standards to ensure that every penny invested in research is well spent, funding only the very highest quality projects. Universities, government and funding bodies use AMRC membership as an indicator of quality research funding.
PSC Support is a National Institute for Health Research (NIHR) non-commercial Partner. This means the studies that we fund may be eligible to access the NIHR Study Support Service which is provided by the NIHR Clinical Research Network. The NIHR Clinical Research Network can now support health and social care research taking place in non-NHS settings, such as studies running in care homes or hospices, or public health research taking place in schools and other community settings. Read the full policy: Eligibility Criteria for NIHR Clinical Research Network Support.
In partnership with your local R&D office, we encourage you to involve your local NIHR Clinical Research Network team in discussions as early as possible when planning your study. This will enable you to fully benefit from the support available through the NIHR Study Support Service.
If your study involves NHS sites in England you will need to apply for Health Research Authority Approval. For guidance on submitting an application please visit: www.hra.nhs.uk.
For each funding application, written peer reviewers (at least three scientific experts and two lay experts) will submit scores and comments to the PSC Support Scientific Review Committee according to set criteria and our Research Strategy.
External reviewers will be selected where the Committee does not have sufficient expertise, or where the grant is £55,000 and above. External reviewers may be selected from the wider research and clinical community, or have special expertise in relation to a funding application. PSC Support will work with the Scientific Review Committee to identify appropriate external reviewers.
The Scientific Review Committee will consider the written peer review comments/scores to agree their recommendations to the Trustees of PSC Support. The Trustees of PSC Support have the final decision regarding funding research projects.
Grants are awarded on the basis of scientific merit and clear relevance to PSC Support’s Research Strategy. The Scientific Review Committee scores the application according to the following criteria:
Scientific Appraisal Criteria Guide
- Relevance of idea or proposal
Refer to PSC Support’s Research Strategy. How does the proposal relate to our charity aims and priorities? - Patient-Relevant outcomes
Is the proposal testing patient-centred outcomes eg quality of life, survival, itching, pain, fatigue, group identification (eg high risk cancer, high risk PSC)/ making progress towards using patient-centred outcomes in the future? - Originality
Does the proposal address an important and valid research question? Also refer to PSC Unanswered Questions. - Realistic costing and value for money
Is the budget realistic, value for money and costs justified? - Relationship to and the volume of research already available in the field
Has the proposal considered relevant PSC literature and developments? - Scientific quality or methodology
What is the quality of the experimental design or method? - Expertise of researchers
Does the research team have the right expertise for the proposed research? - Use of animals in research
Funding applications involving the use of animals must comply with the law and support the principle of the 3Rs to refine, reduce and replace the use of animals in research. PSC Support supports the AMRC Statement on Animal Research. Our expectations for responsible use of animals are set out in the document Responsibility in the use of animals in bioscience research.
Lay Appraisal Criteria Guide
The research grant applications are considered by lay reviewers (usually patients) as well as by scientific experts. Lay reviewers will appraise the application based on the importance of this research and the potential difference this could make to people affected by PSC, and the progress this research could make towards answering an ‘unanswered research question’.
Patients are involved in appraising research applications because PSC Support places great value on the patient voice. For this reason, there is no word limit on the lay summary section of the application form. While patients might not fully comprehend the technical and scientific aspects of the project, they are interested in what the project entails and we 'get' that it takes more words when you explain something technical!
- Importance of this research to people with PSC
Do you think this research is important to people with PSC? - Makes a positive difference
How optimistic are you that this research will make a positive difference to people with PSC? - Makes significant progress towards an ‘unanswered question’ in PSC
Do you think this research is answering one of our ‘unanswered questions’ or making progress to answering one? - Lay summary's ease of understanding
How easy is the Lay Summary to understand?
Success rates of eligible applications are as follows:
| Year | Number of Applications | Number funded | Success Rate |
| 2015/16 | 3 | 2 | 67% |
| 2016/17 | 4 | 2 | 50% |
| 2017/18 | 4 | 3 | 75% |
| 2018/19 | 1 | 1 | 100% |
| 2019/20 | 4 | 3 | 75% |
| 2020/21 | COVID | COVID | 0% |
| 2021/22 | 1 | 1 | 100% |
| 2022/23 | 1 | 1 | 100% |
| 2023/24 | 8 | 4 | 50% |
This Code of Conduct applies to all members of the PSC Support Scientific Review Committee (SRC), external reviewers, applicants and staff/volunteers of PSC Support involved in a funding round.
- Maintaining confidentiality is essential for safeguarding the exchange of scientific opinions and assessments.
- Details of research applications and related correspondence, and SRC meetings’ papers are strictly confidential and must be kept secure and not disseminated to or discussed with others outside the review process.
- External reviewers and SRC members must agree to treat all details of applications and their outcomes as confidential.
- External reviewers and SRC members must agree not to input content from our confidential funding applications or reviews into, or use, generative AI tools to develop their peer review critiques. Our peer reviewers are selected for their expertise and experience in their field and we value their unique perspectives.
- Applicants can expect that details of their applications will not be disclosed to those outside the review process by PSC Support.
- SRC members and external reviewers can expect appraisals of their applications to be treated in confidence by PSC Support. When we inform applicants of the outcome of their applications, we provide feedback based on reviewers’ comments, and may also summarise the conclusion reached by the SRC. Information that might identify the comments of individual reviewers is never revealed.
- SRC members and external reviewers will be notified by PSC Support of the final decisions (made by Trustees) of any applications they have reviewed.
- Any member of the SRC who declares a conflict of interest (including a current association with an institution applying for a grant) will not participate in that activity, they will not receive the application(s) and will not be present for discussion of the application(s) at the SRC meeting.
- Applicants must not make direct contact with members of the SRC about their application and vice versa.
ResPol1.3
PSC Support follows the AMRC principles in the use of Animals in Research
We support the AMRC statement on animal research.
Use of Animals Policy
PSC Support appreciates that research using animals plays an important role in the research process and we support the principle of using animals when it is necessary
- to advance the understanding of primary sclerosing cholangitis and associated conditions.
- to develop treatment when there is no alternative that can be used to find out the same information without using animals.
PSC Support will only fund research involving live animals when this is essential to the outcome of the research, there is no alternative method of obtaining the data, and when pain and distress to animals is minimised.
We use expert peer review to ensure that we fund only high quality research where the benefits to human health outweigh any harm to animals. The use of animals must be fully justified in all applications for research grants.
PSC Support requires that all experiments should be carried out with due concern for the welfare of the animals, and using the minimum number necessary to provide clear data in well-designed experiments.
Individuals working on experiments using animals must have a personal licence and the institution must also have an establishment licence.
High standards of animal welfare are important. These both minimise discomfort for the animals involved and enable researchers to get reliable results. We only fund research which complies with the law and support the principle of the 3Rs to refine, reduce and replace the use of animals in research:
- Replace the use of animals with alternative techniques, or avoid the use of animals altogether
- Refine the way experiments are carried out, to make sure animals suffer as little as possible. This includes better housing and improvements to procedures which minimise pain and suffering and/or improve animal welfare.
- Reduce the number of animals used to a minimum by seeking ways to find out information from fewer animals or more information from the same number of animals.
PSC Support accepts that not all of the public can accept research using animals.
Our PSC Support Agreement for grant research awards specifically refer to the ethical use of animals. Our requirements are that:
1.1. A project involving the use of animals may not be commenced in the absence of Home Office licences covering all relevant institutions the researchers and the research activity. Acceptance of an award constitutes confirmation that any necessary consent including Home Office approval under the Animals (Scientific Procedures) Act 1986 or other relevant legislation has been obtained. Grant holders are expected to adopt procedures and techniques which minimise the use of animals.
1.2. Research using animals plays an important role in the research process and PSC SUPPORT supports the principle of using animals when it is necessary to advance the understanding of Primary Sclerosing Cholangitis and associated conditions to develop treatment when there is no alternative that can be used to find out the same information without using animals. Expert peer review is used to ensure that we fund only high quality research where the benefits to human health outweigh any harm to animals. PSC SUPPORT supports the Association of Medical Research Charities statement on animal research.
1.3. All experimental programmes supported by PSC Support must only use animals were there are no alternatives.
1.4. Experiments using animals funded by the PSC Support must:
1.4.1. use the simplest possible, or least sentient, species of animal
1.4.2. ensure that distress and suffering are avoided wherever possible
1.4.3. employ an appropriate design and use the minimum number of animals consistent with ensuring that the scientific objectives will be met
1.5. See the NC3Rs website for further information and guidance.
1.6. All grant holders using animals must implement the principles in the cross-funder guidance Responsibility in the Use of Animals in Bioscience Research.
1.7. Grant holders using non-human primates must comply with the NC3Rs guidelines Primate Accommodation, Care and Use.
1.8. Grant holders should make use of the ARRIVE guidelines when designing their experiments, and ensure that they report animal-based studies in accordance with the ARRIVE guidelines as far as possible, taking into account the specific editorial policies of the journal concerned.
ResPol4.2
PSC Support follows the AMRC Statement on Supporting Research in Universities
As a member of AMRC, PSC Support endorses the principles of the AMRC Statement on Supporting Research in Universities.
Key principles
- AMRC charities remain committed to supporting research and the careers of talented researchers in UK universities;
- AMRC believes that government is primarily responsible for provision of underpinning funding for the UK biomedical science base;
- Where it is within their charitable objects and appropriate for them to do so, AMRC encourages its member charities to contribute towards a sustainable science base in universities through the support of longer-term funding for people and facilities;
- Universities should understand the full economic costs of proposed research facilities or activities when seeking financial support from charities;
- Charities will not normally meet the full economic costs of the research they are supporting, especially for responsive mode funding calls;
- Universities will be expected to contribute resources from QR, including the charity support element, in order to meet the full costs of research in partnership with charities;
- Charities will not contribute a percentage overhead towards general university infrastructure;
- Charities are unlikely to respond positively to demands to fund research activity on the basis of a fixed percentage of research costs (as is the case for other funders including the Research Councils); and
- AMRC will work with member charities, government and universities to develop new models of partnership funding that will enable charities of all sizes to develop their commitment to research in a sustainable way.
ResPol5.1
As a member of the AMRC, PSC Support follows the Research Funders Policy Group joint statement on the use of generative AI tools in funding applications and assessment.
Generative AI tools offer potential benefits for research but also challenges and risks. The joint statement sets consistent standards on generative AI tools in funding applications and assessment across research funding organisations in the UK. It makes clear that AI technology should not be used for peer review and that, if used in other contexts, such as preparing funding applications, it must be clearly cited and acknowledged.
The following list of questions is taken from responses in our PSC Support Surveys.
Questions about why people get PSC
What is the cause of PSC?
Can PSC be prevented?
What is PSC doing to me?
Who gets PSC?
Why is there such variability between PSCers?
What environmental factors are involved in PSC?
Questions about curing or halting PSC
Can you cure me?
Can you halt the progression on PSC?
Questions about diagnosis, prognosis and identifying PSC risks
How can PSC be diagnosed early?
Why does PSC return after transplant in some people?
What is my prognosis?
Why does PSC progress quickly in some people?
Will I need a transplant?
Identify warning signals that my PSC will be aggressive.
Identify warning signals that I may get a cancer.
Questions about symptom treatment
Can you treat my fatigue and other symptoms?
Does exercise help fatigue in PSC?
Questions about helping ourselves
What can I do to stay healthy?
Should I eat a special diet?
Questions about PSC and other diseases
How is PSC linked to Inflammatory Bowel Disease?
How is PSC linked to other diseases, especially autoimmune diseases?
Questions about therapies
Do antibiotics work in PSC?
Does bacterial therapy work in PSC?
What does Urso do?
Questions about clinical care
How can I get a doctor that understands PSC?
Questions about genetics
How are genetics involved in PSC?
Are members of my family likely to have PSC?
Can you screen for PSC?
Where there is a potential conflict of interest, the reviewer, member or Trustee shall not be party to any decision or have sight of the application form. Please see our Conflicts of Interest Policy.
Research costs can be direct and indirect, and charities do not pay indirect costs. We ask that you calculate your costings using AcoRD.
If you have any questions regarding our funding, please get in touch.
Applicants sometimes contact us to ask informally about the appropriateness of their application and seek advice about our remit. Such enquiries are dealt with by charity staff who are not scientific experts and will only advise if an application isn’t suitable for funding when it is clearly outside our remit. Charity staff will not comment on likelihood of securing funding from PSC Support because all applications must go through the independent peer review process prior to any decisions about funding.

