Aspirin to Prevent Cancer in people with PSC
PSC Support Survey Results
Date published: 10 June 2019
Dr Simon Rushbrook (Norfolk and Norwich University Hospital), Professor Shahid Khan (Imperial College, London) and Martine Walmsley (PSC Support) developed the survey questions in order to gauge interest and attitudes towards a proposed study using aspirin to try to prevent cancers associated with PSC.
The survey ran from 18 Feb to 30 April 2019 and 190 people took part, from:
- the PSC Support online Facebook support group and
- recipients of the PSC Support Research news bulletin in the UK.
This survey represents a snapshot of attitudes of people with PSC. Thank you to everyone who took the time to give their views in this survey.
This survey demonstrates that reducing the risk of cancer is important to people affected by PSC and they are interested in helping reduce the risks of PSC-related cancers.
Demographics
The majority of people who took part in our survey were from the UK.
| Country | Count |
| United Kingdom | 159 |
| Ireland | 9 |
| United States | 8 |
| Australia | 2 |
| Germany | 2 |
| Sweden | 2 |
| Armenia | 2 |
| Country | Count |
| Belgium | 1 |
| Canada | 1 |
| Israel | 1 |
| Netherlands | 1 |
| New Zealand | 1 |
| Romania | 1 |
| Serbia | 1 |
All but six people had PSC or had had a transplant for PSC.
The age of respondents ranged from 13 to 77 years.
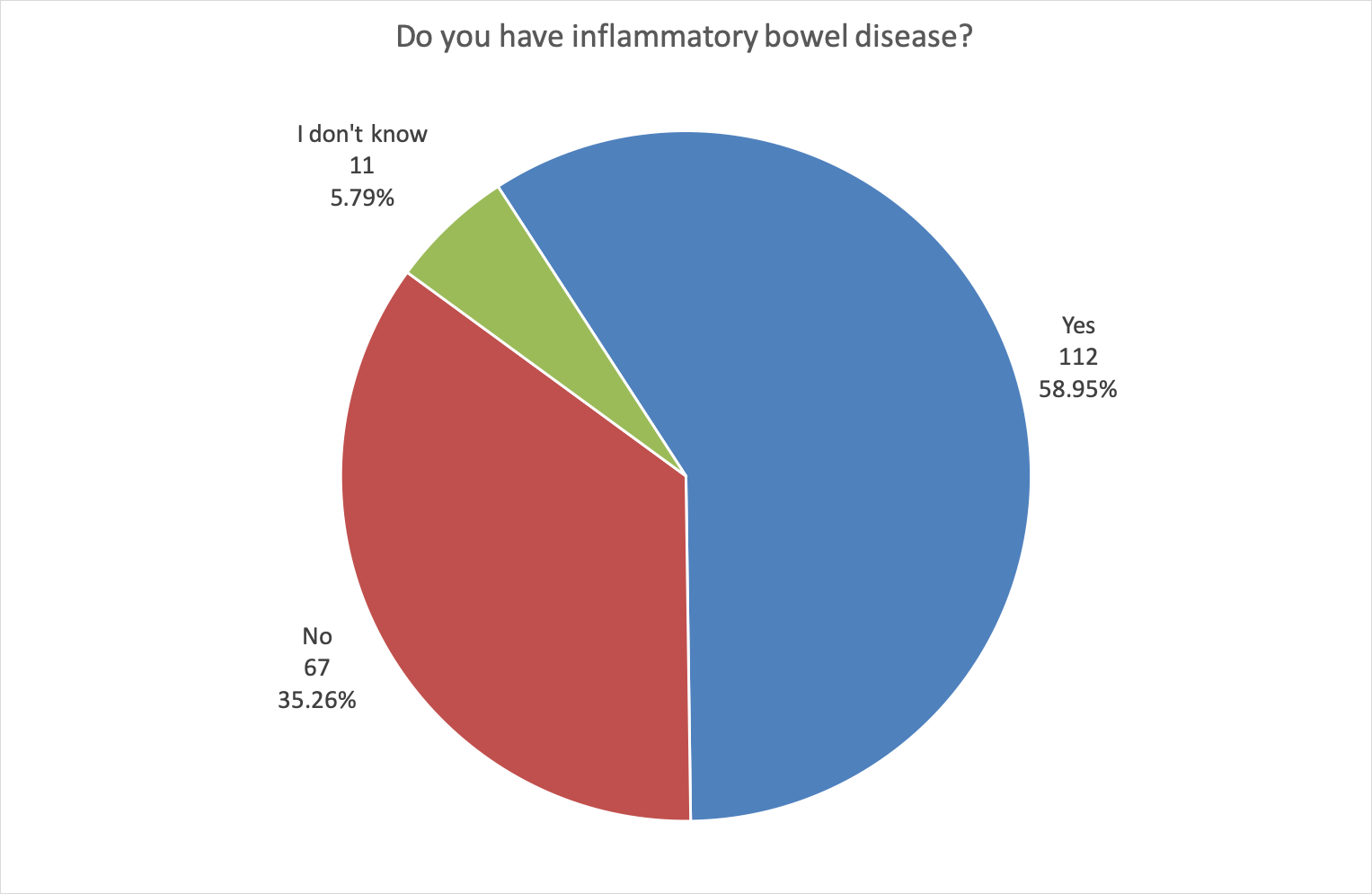
As expected, many (58.95%) also had inflammatory bowel disease (IBD).
Two-thirds of respondents were female and a third were male, perhaps reflecting the make-up of those accessing and engaging with PSC Support.
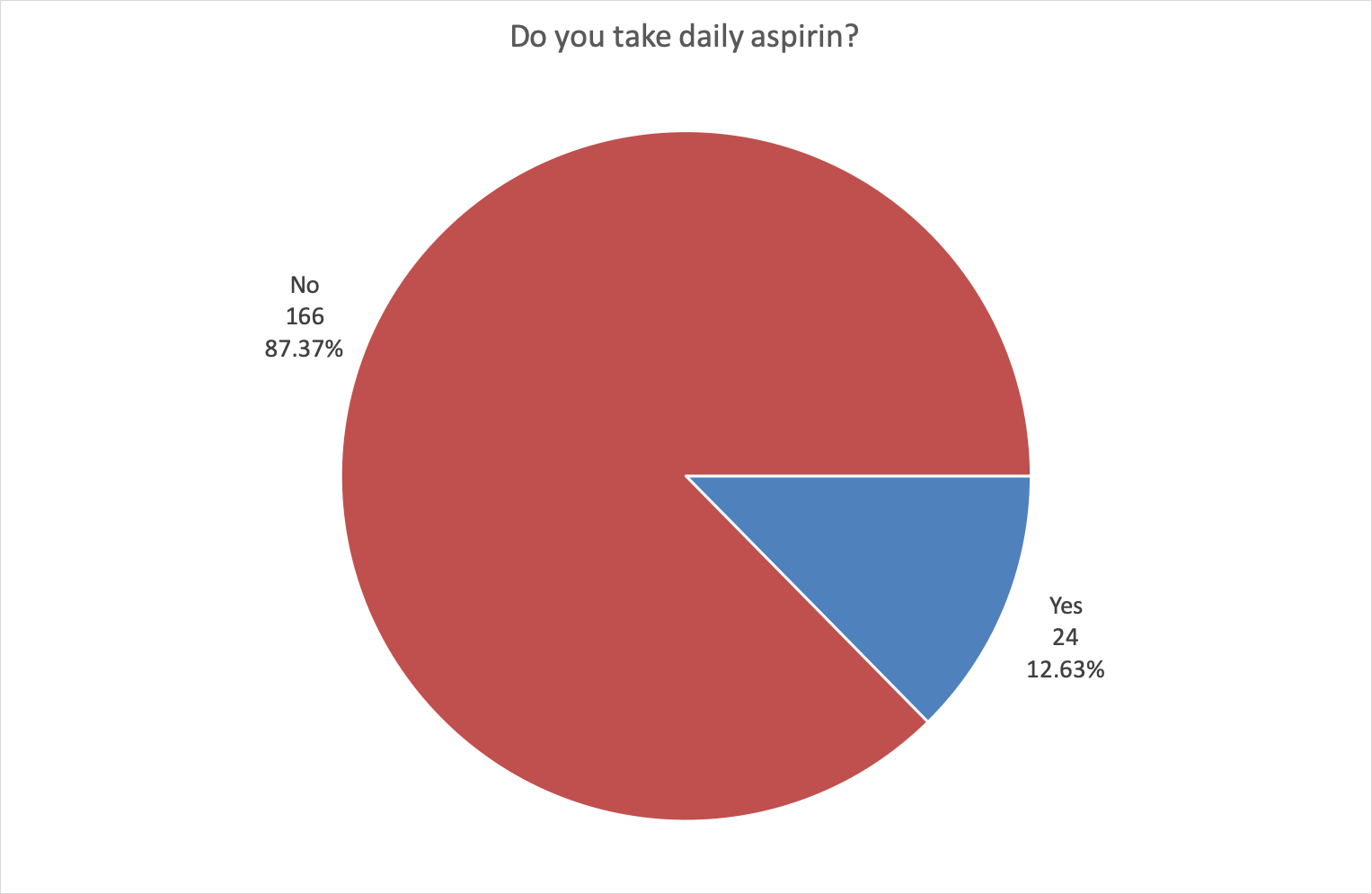
12.63% of respondents already took daily aspirin.
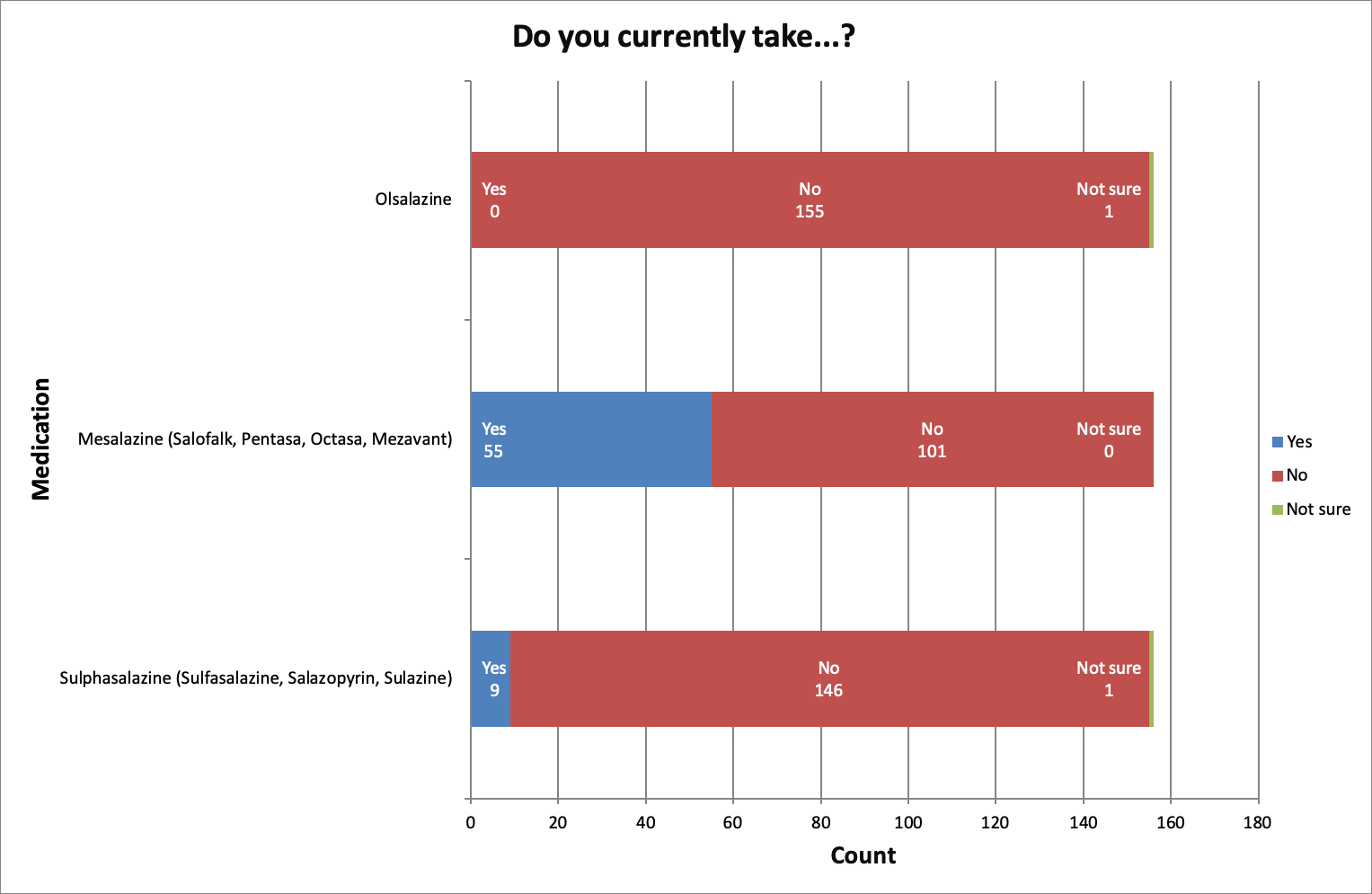
The most frequently taken medication out of sulphasalazine, mesalazine and olsalazine was mesalazine.
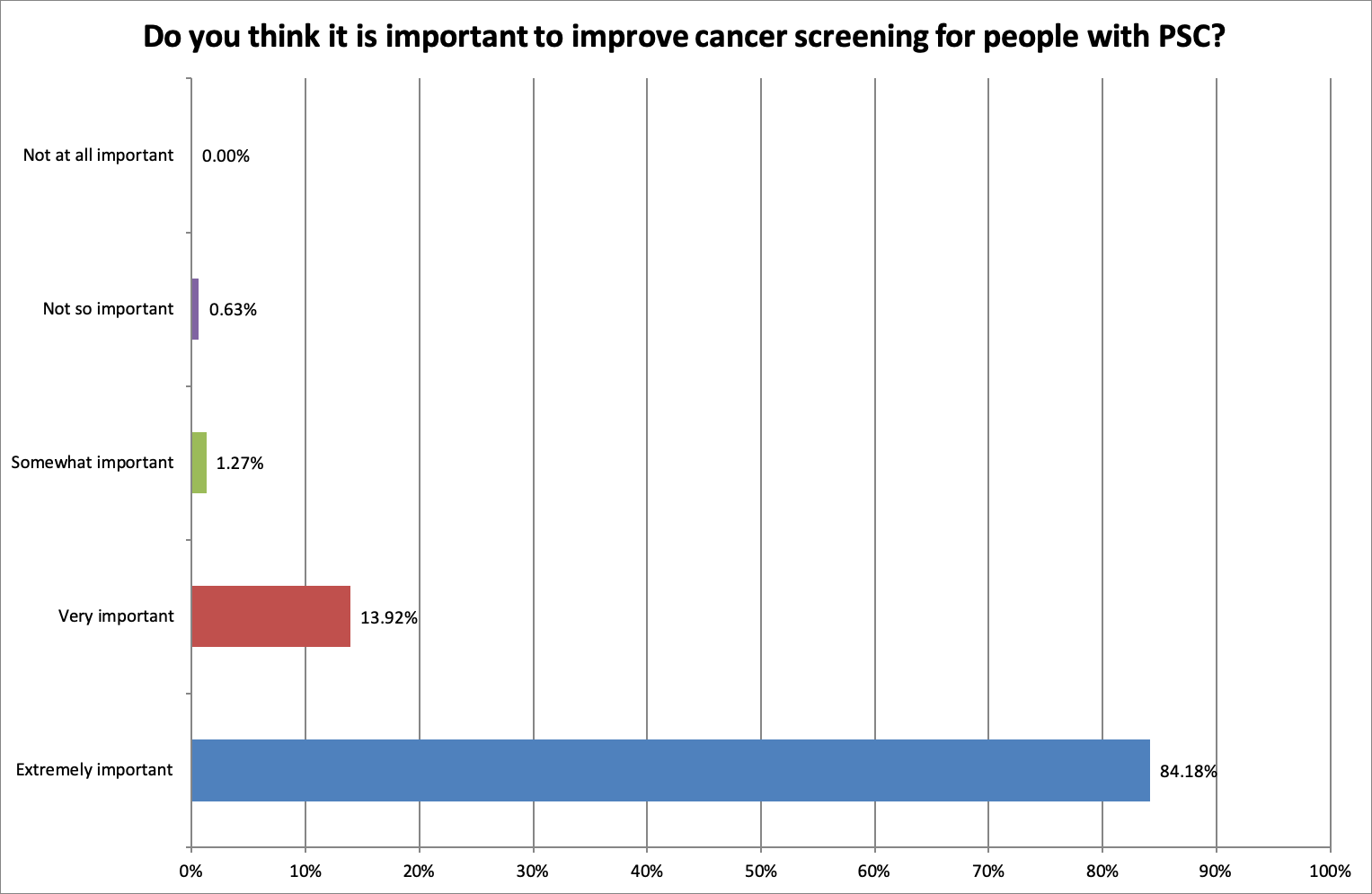
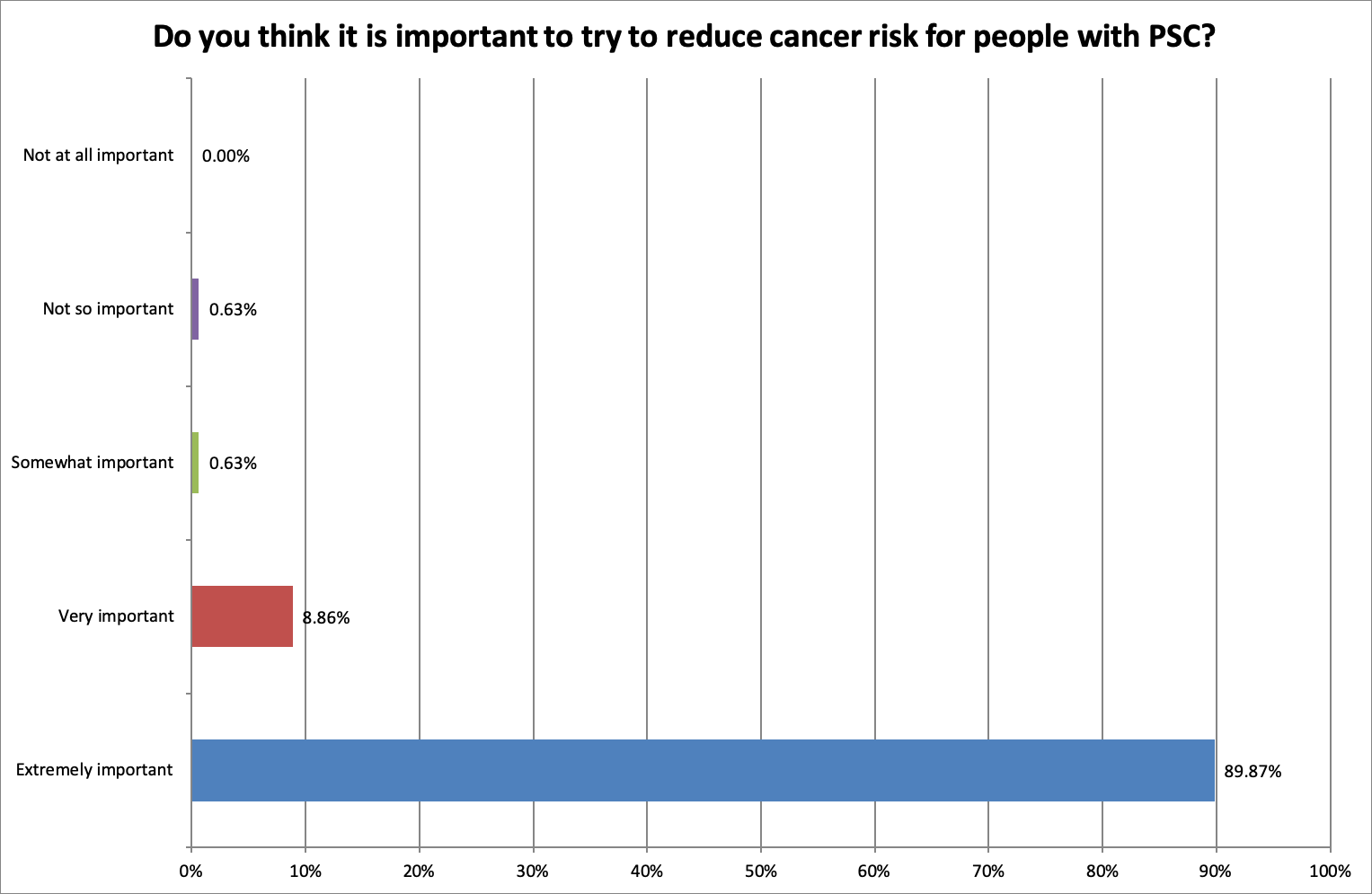
Is it important to try to reduce the cancer risk for people with PSC?
As anticipated, the answer was overwhelmingly yes:
89.87% of respondents felt it was extremely important to reduce the cancer risk and improve screening (84.18%) for people with PSC.

90.51% said that it was extremely important to look for new ways to detect cancer.
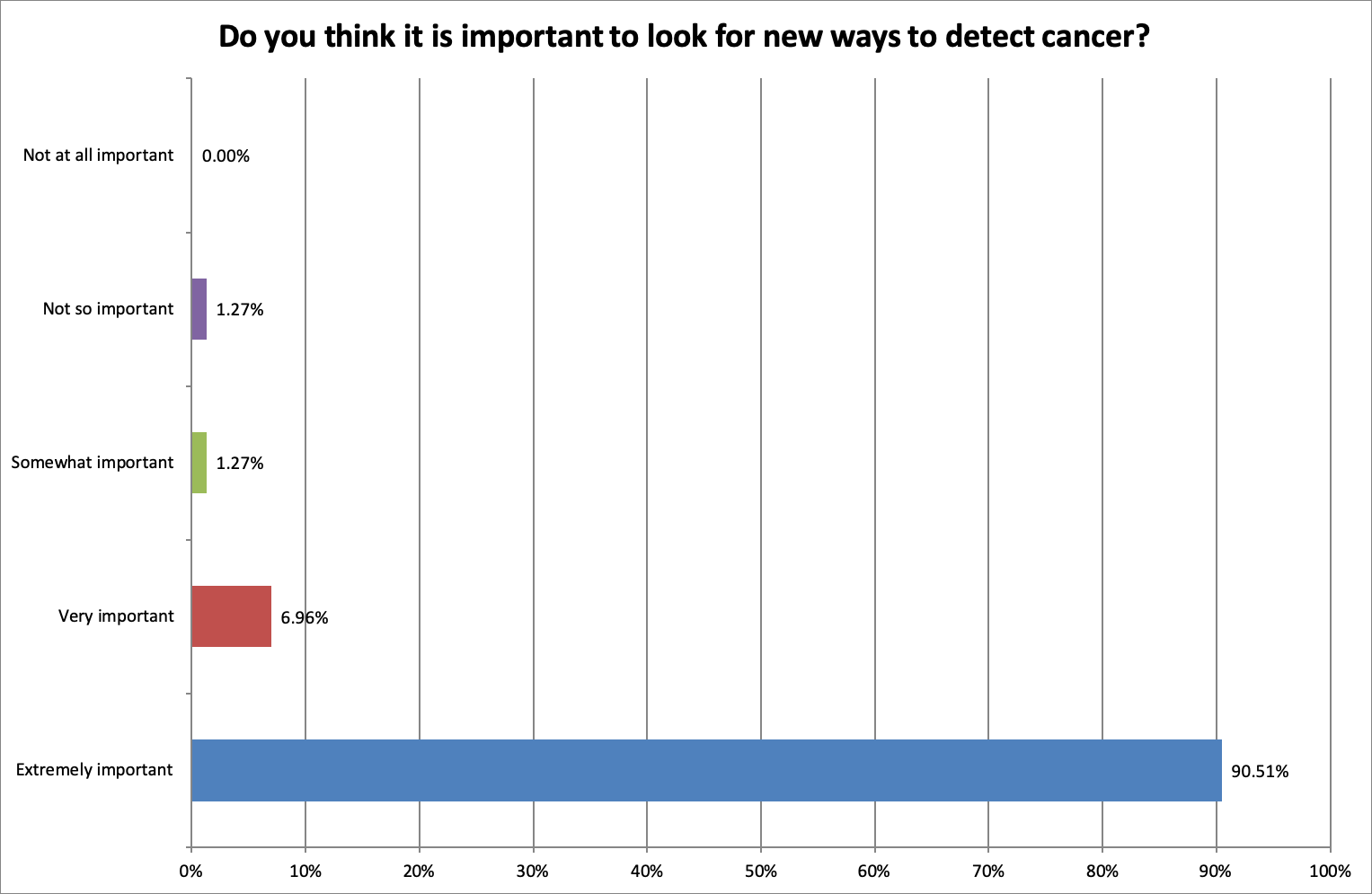
One respondent said, “PSC is bad enough. Cancer is even worse, so if a cheap safe drug proves to reduce the higher than normal risk to at best a normal risk, it's got to be worth looking into.”
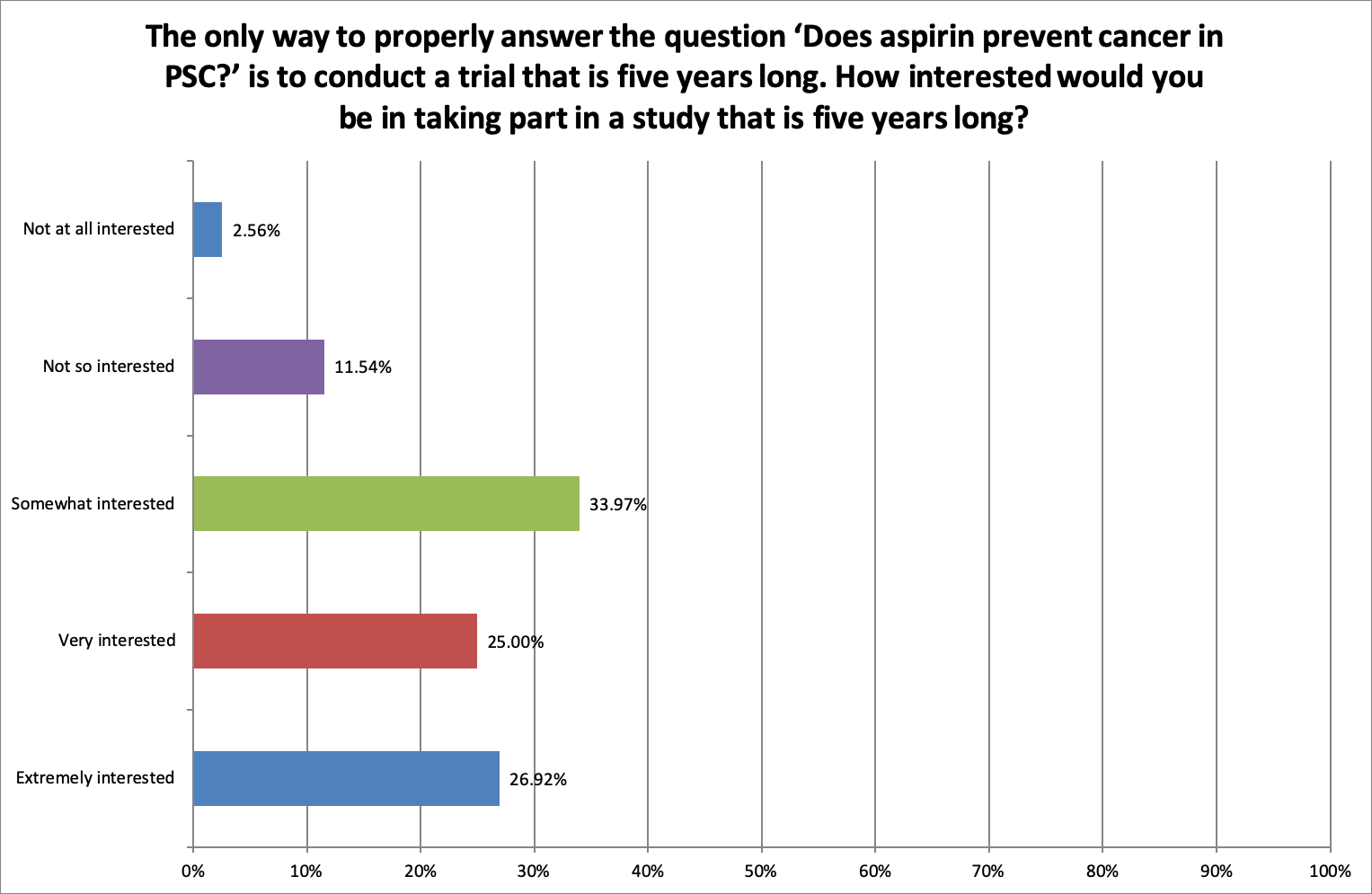
Taking part in a study for five years
Most respondents (85.89%) were ‘somewhat’, ‘very’ or ‘extremely interested’ in taking part in a five-year study to ask ‘Does aspirin prevent cancer in PSC?’ with only 14.1% selecting ‘Not so interested’ or ‘Not at all interested’.
This, combined with other answers in this survey, suggests that the duration of the trial is not so important to patients when an important question is being addressed or when additional monitoring is offered.
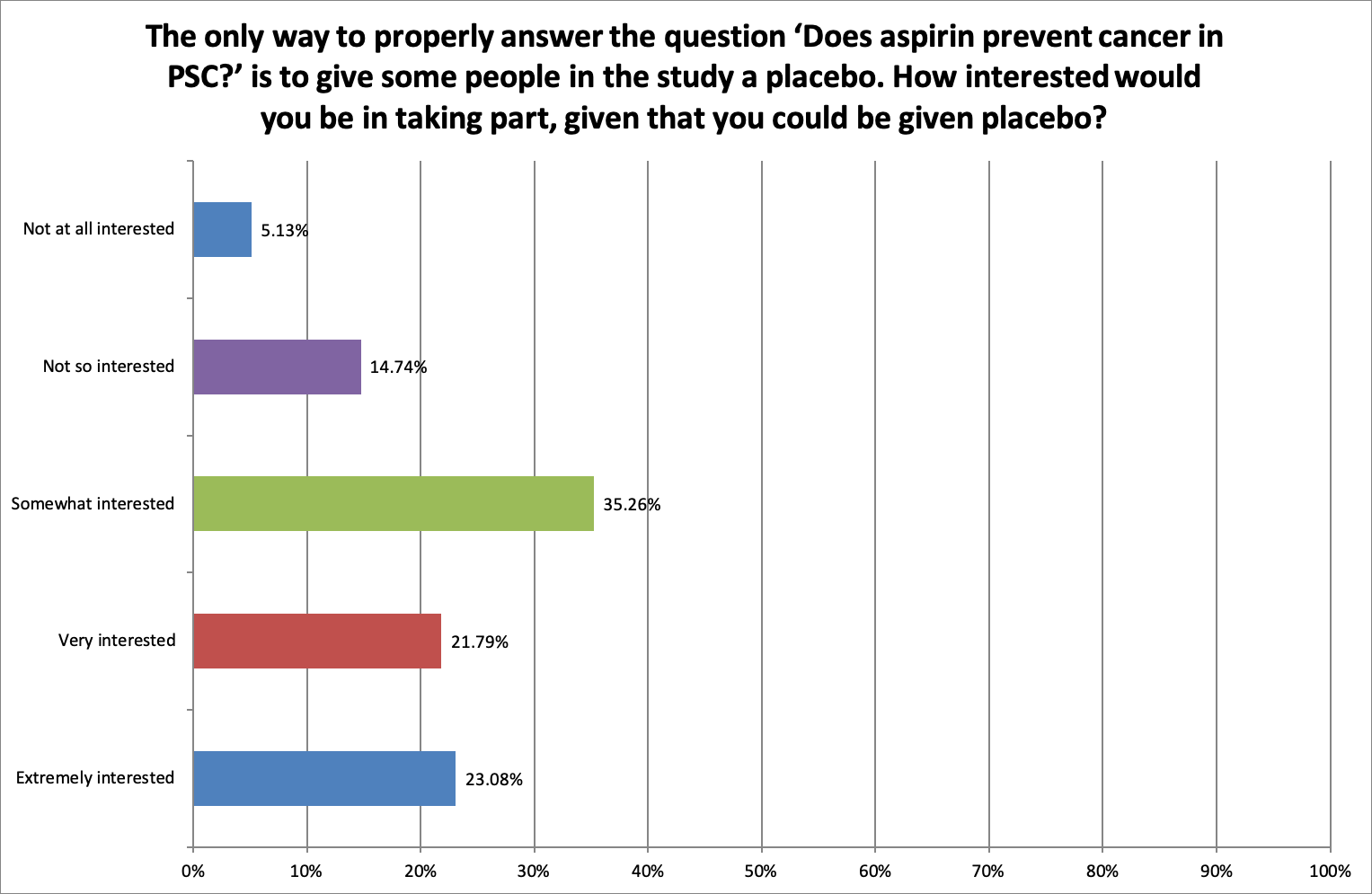
Being in the placebo arm of a study
Most respondents (80.13%) were ‘somewhat’, ‘very’ or ‘extremely interested’ in taking part in the study despite the fact that they could be given a placebo drug.
Only 19.87% said they were ‘Not so interested’ or ‘Not at all interested’ in taking part in a study to prevent cancer where they might be given a placebo.
Additional scans and monitoring for cancer make trial participation more likely
In order to find out if having more scans than current standard of care would put people off taking part, we asked, ‘Would having more detailed scans at more regular intervals to try to detect more cancers, earlier, make you more or less likely to take part?’
92.31% thought it was extremely or very important to have more detailed scans at more regular intervals to try to detect more cancers, earlier.
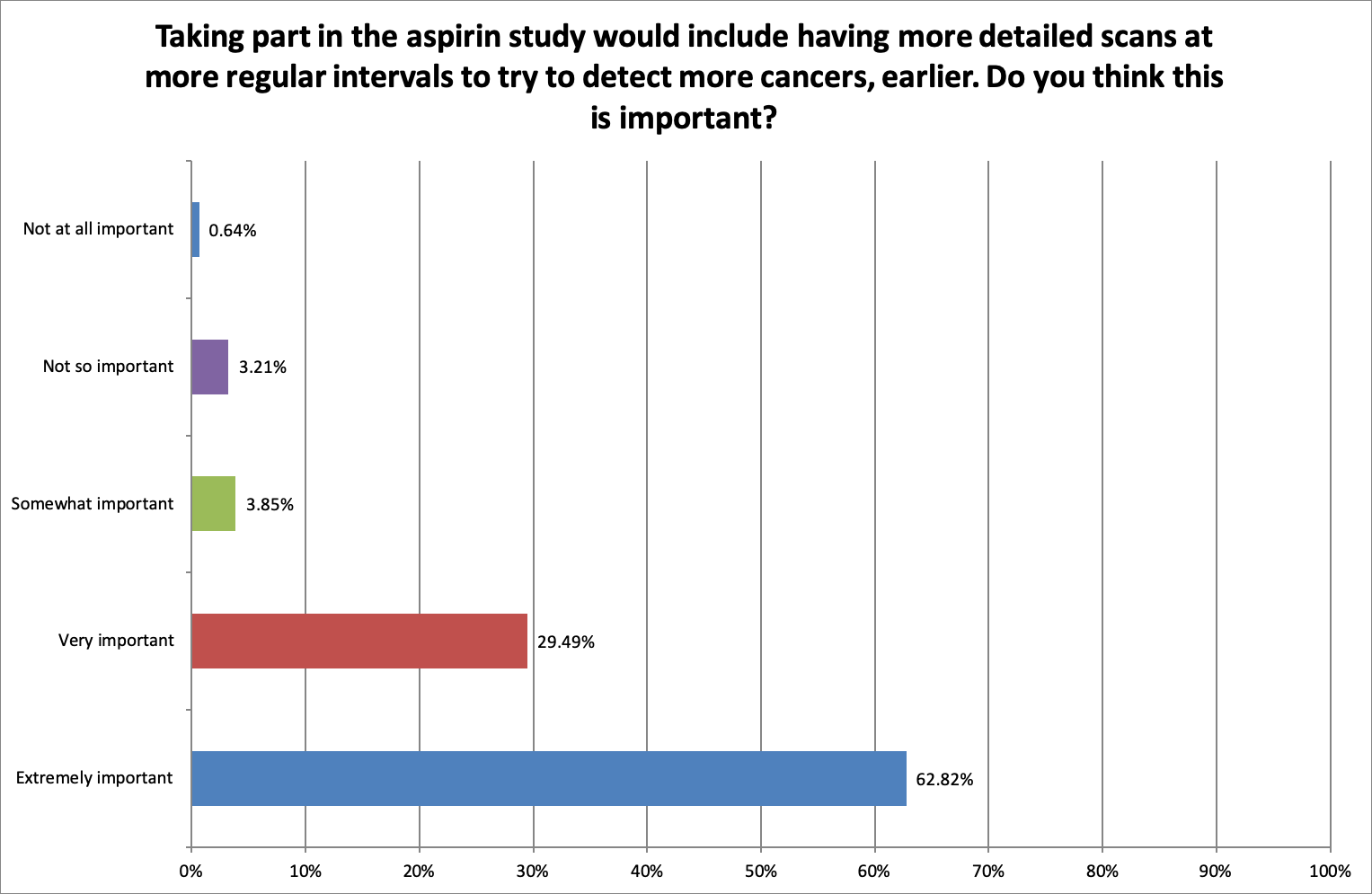
66.01% said having more detailed scans, more frequently would make them more likely to take part. 29.40% said that additional scans would not make a difference and only 4.49% of respondents felt it would make them less likely to take part.
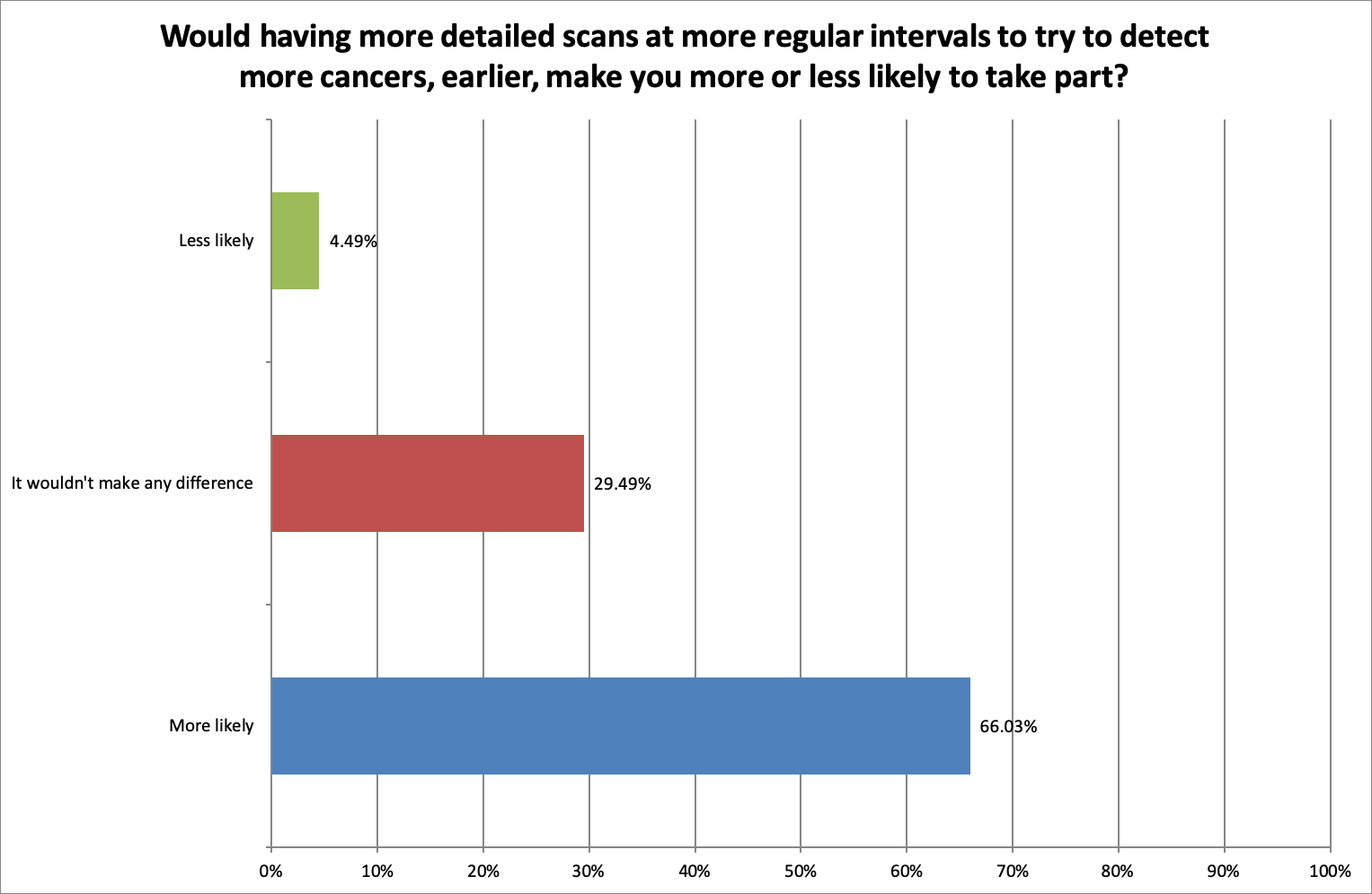
Additional scans and monitoring was seen in a positive light. Comments included:
“...can only answer as the mum of a 17 year old just diagnosed with PSC and with Crohn’s disease 4 years ago. But I would really approve enhanced and more frequent screening.”
“People will join this trial because of the regular scans and monitoring.”
“I confess that part of the attraction of this study is the closer monitoring for various cancers. Nonetheless, I would still be interested if this was not the case.”
Patients want to take part in clinical trials
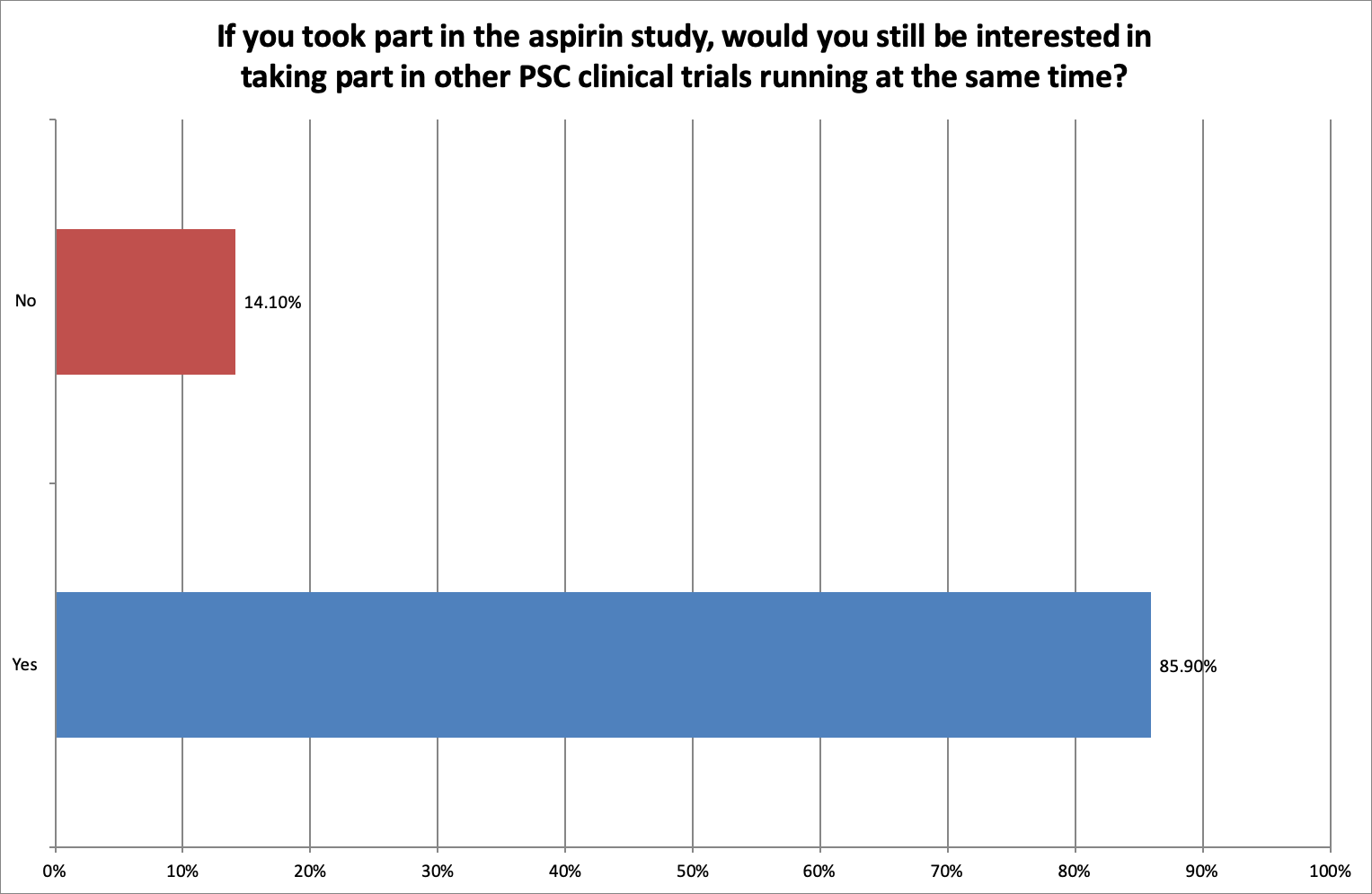
PSC is a rare disease, and finding and recruiting enough patients is critical in all PSC studies. We wanted to understand the potential impact this may have on recruitment to other studies.
85.9% of respondents said they would still be interested in taking part in other studies while they took part in the aspirin study.
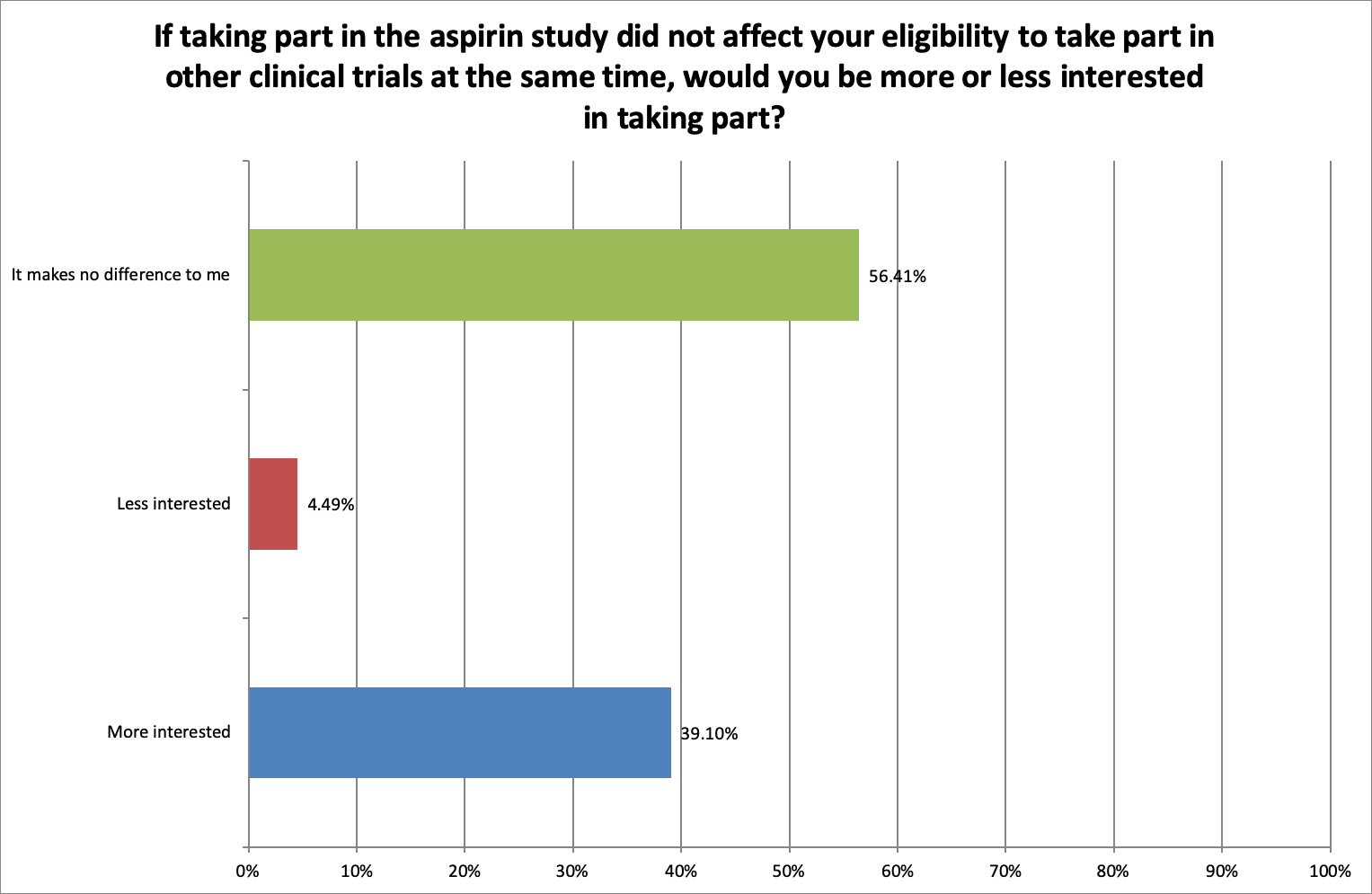
When asked, ‘If taking part in the aspirin study did not affect your eligibility to take part in other clinical trials at the same time, would you be more or less interested in taking part?’ 39.1% said they would be more interested, 4.49% said they would be less interested, and 56.41% said that it would make no difference, indicating an overwhelmingly positive attitude to participating in the aspirin and other research.
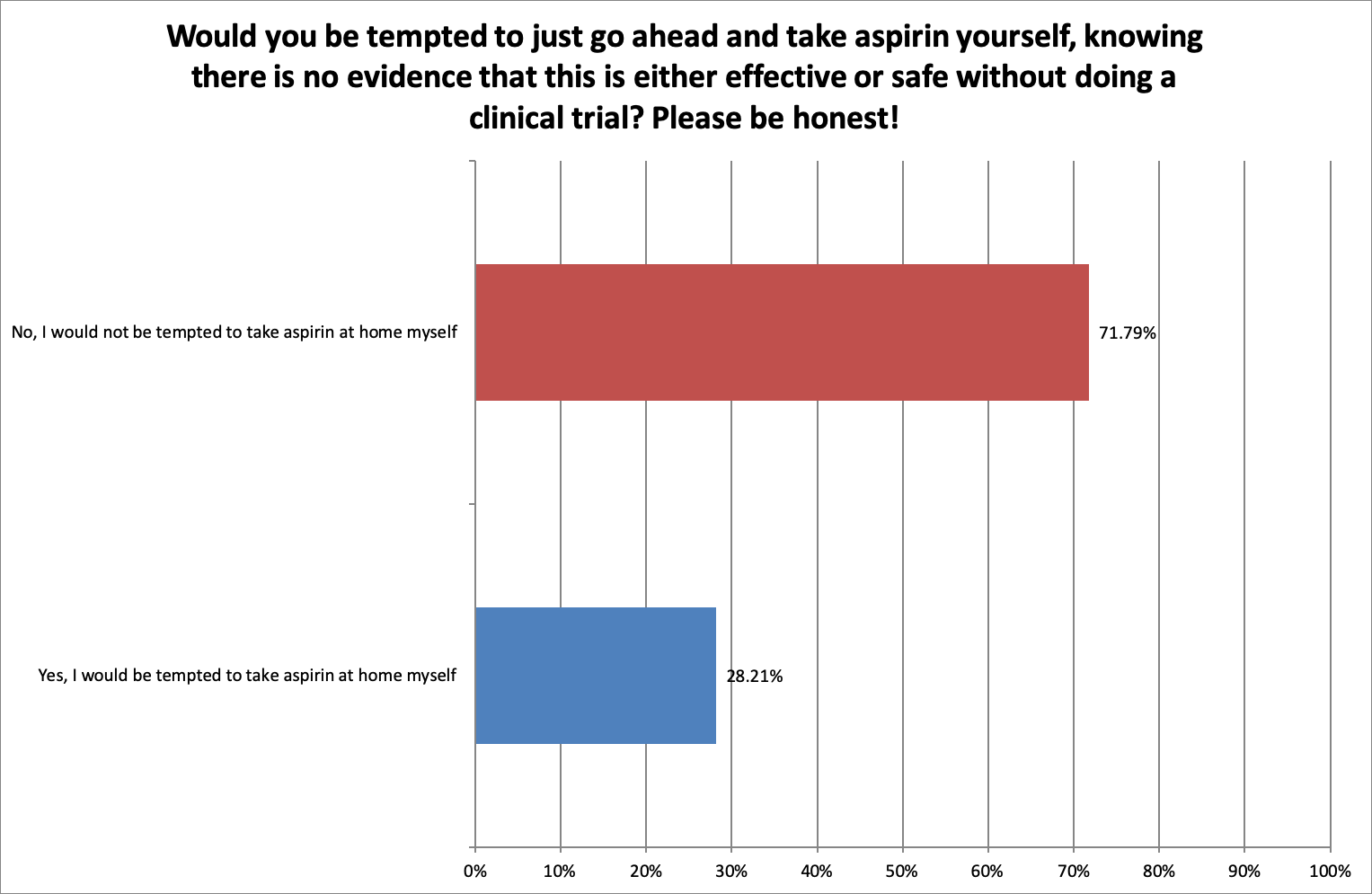
Self-medicating daily aspirin
Nearly a third of the respondents said they would be tempted to take aspirin themselves, knowing there is no evidence that this is either effective or safe without doing a clinical trial.
However, the comments indicated that respondents were in fact conscious of safety, well-informed about aspirin, aware of the potential risks, and wanted to know more.
“I'm not receiving regular screening and feel frightened by thought of cancer so I might be tempted [to self-medicate with aspirin] but won't do this as wouldn't want to jeopardise chance of trial selection.”
“Without the guidance of a trial and the extra scans etc that would come with the trial, the risks associated with taking [self-medicating] aspirin would be too significant to risk.”
Final comments
Reducing the risk of cancer is important to people affected by PSC and they are interested in helping reduce the risks of PSC-related cancers.
Clear and comprehensive patient information would be required for a study using a widely available, easily accessible medication such as aspirin. Respondents wanted to understand the risks involved by taking aspirin. Respondents were not put off by long trial duration, or the risk of being in the placebo arm, although this did reduce interest slightly. Undergoing additional scans and monitoring made people more interested in trial participation in a study to reduce cancer risk.
One respondent summed-up the attitudes expressed in the survey:
“Cholangiocarcinoma is one of our biggest fears - I fear it over any other aspect of PSC, more so even than going through transplant and added to the fact that in the UK we don’t even get a chance at transplant if cancer is discovered. So yes, I would welcome a trial until something that improves our chances…”
